For Cellistic, a leading Contract Development and Manufacturing Organization (CDMO), achieving its strategic vision hinged on building the world’s first GMP-certified, purpose-built facility for human-induced pluripotent stem cell (iPSC) therapies. Completing this project within 16 months, starting from a blank sheet of paper, was a key enabler in realizing their goals.
iPSCs, often described as a cornerstone of modern biomedical research, are adult cells reprogrammed to behave like embryonic stem cells. This technology underpins allogeneic therapies - treatments that leverage universally compatible cells to combat a host of diseases, including cancer, autoimmune disorders, and degenerative conditions like Parkinson’s disease.
The new facility would not only be the first of its kind in the world, it also had to be delivered on a tight, 16-month timeline. With no previously built facilities to model this one on, Cellistic needed a design and construction partner with deep enough process knowledge to build one exactly right the first time.
But there’s more: It had to be adaptable for future processes, since CDMOs like Cellistic need flexible setups to accommodate their end clients’ diverse requirements.
A high-stakes project
From a business standpoint, the stakes could not have been higher. For a CDMO, it’s all about attracting clients. Cellistic needed to secure contracts with companies that would require GMP-grade facilities for their clinical trial materials.
Potential clients would only agree to sign on if Cellistic could ensure that the facility would be validated and operational by a designated date. If the facility was not ready on schedule, clients may opt to seek alternatives.
In short: Cellistic needed a partner they could absolutely trust to translate their process into a functional facility, keeping future flexibility in mind. They needed a lean approach and a team that understood the variability in cell and gene therapy requirements. And they needed it fast, because their whole business just might depend on it.
Fortunately for Cellistic, doing what has never been done before – fast – is our calling card. Their decision to trust our capacity to deliver the required facility on time proved to be beneficial.
A Facility Built for Speed and Precision
At VILS, we know that the key to everything is always understanding the client’s needs deeply. Our task is always to build a fit-for-purpose facility without overengineering. Excessive engineering not only elevates both capital and operational costs but can also undermine the overall business case.
“VILS helped us map out the process from the start,” said Cellistic CTO and founder Stefan Braam. “They created a functional design that aligns perfectly with our goals, enabling us to accelerate allogeneic cell therapies to patients faster.”
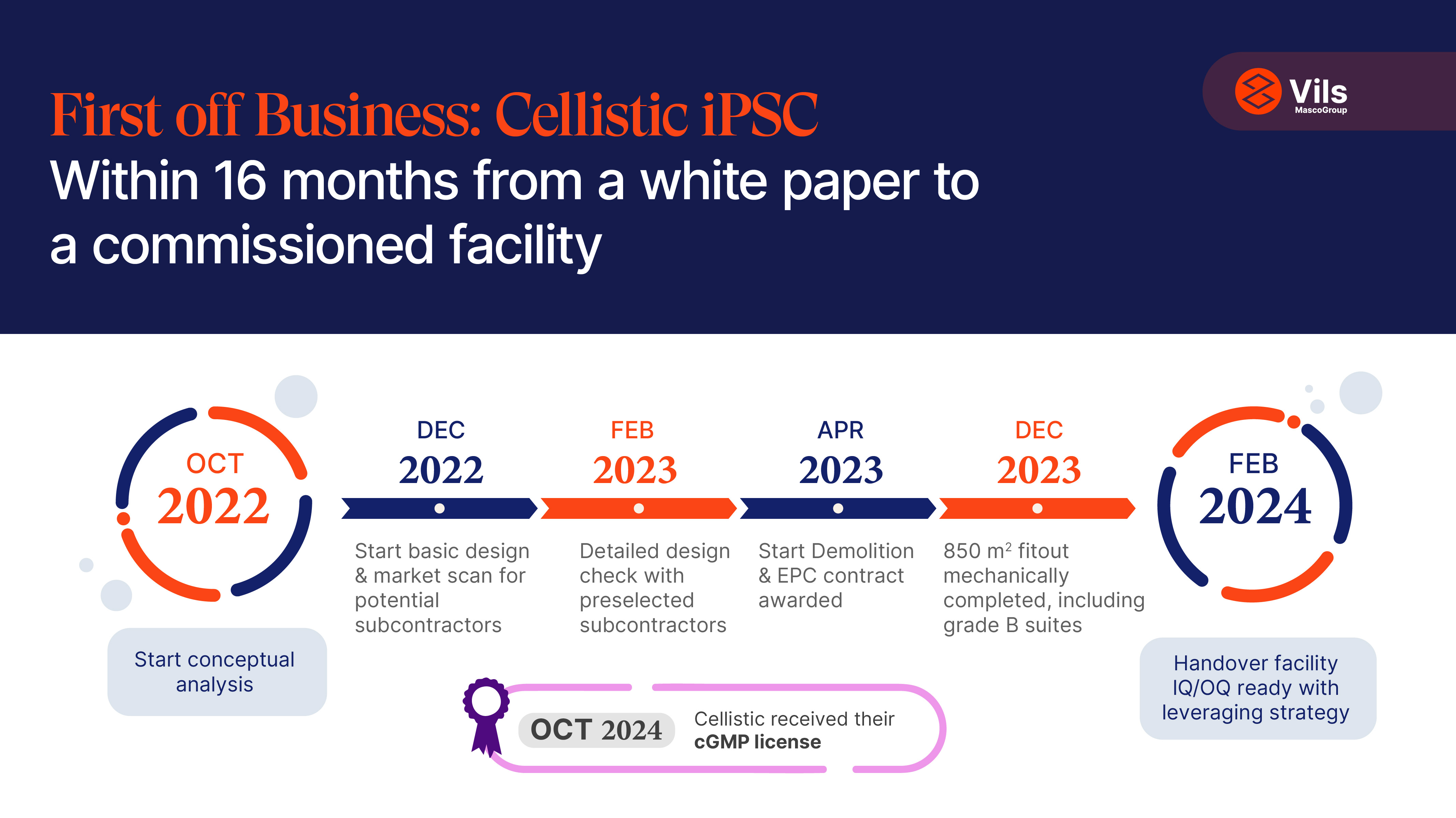
This facility, with its capacity to manufacture hematopoietic stem cells and derivative cell types such as NK cells, macrophages, and T cells, is pivotal to Cellistic’s mission. For a company relying on strict timelines to meet client commitments, this project’s timely success was business-critical.
“From design to build, this was an ambitious project,” said Cellistic VP of Operations Vincent Mancuso. “Throughout, we collaborated closely to address challenges and deliver on time and in full.”
Trust and Transparency: The Cornerstones of Success
Indeed, the collaboration between VILS and Cellistic wasn’t just a technical achievement – it was a masterclass in building trust, and typical of the way we work. We ask clients to balance flexibility and cost, for example. We tell them the truth. And we challenge them to define the real capacities needed for their treatments.
If they insist on planning for every possible scenario, costs skyrocket. It’s like buying a house too big for your current needs - it’s expensive and unnecessary.
The approach was exactly what Cellistic wanted and needed.
As Cellistic CEO Gustavo Mahler noted, open and honest communication between the teams was instrumental. “VILS provided us with clear options to navigate roadblocks while staying on budget and within timelines. The rules of engagement were simple: be transparent and work together.”
This no-nonsense approach, paired with our expertise in process-based design, ensured not only the facility’s rapid completion but also its approval as a GMP-compliant operation. The design process, rooted in deep understanding of Cellistic’s manufacturing needs, enabled streamlined workflows and precise documentation—key factors for regulatory success.
Related story: Building the Future of mRNA Therapies





ATMP and Linked Industries
Cutting-edge therapies bring new hope
Cutting-edge therapies bring new hope